One of the earliest studies was conducted by Richard C. Schoonmaker et. al. (Ref. 42), who studied the vaporization of GaN by weight loss and torsion effusion techniques. They found that in the temperature range around 900°C GaN vaporizes by decomposition to Ga_(g) and N_(2 (g)) in contrast to earlier suggestions that GaN vaporizes as GaN_(n (g)) in a temperature range around 1000°C. Their results suggested that at that temperature the decomposition rate of GaN is very slow. Their most interesting result though is the observation that the rate of vaporization of GaN is markedly enhanced in the presence of Ga or In, and they concluded that these metals serve as a catalysis for the vaporization of GaN by providing an alternate path of lower activation energy. No quantitative data were given though due to the limitations of their apparatus to deal with the low vapor pressure of GaN at around 900°C.
A more detailed study was conducted by Z. A. Munir and A. W. Searcy (Ref. 43) at about the same time. They confirmed that GaN decomposed by the reaction
(63) ![]()
and the reaction rate was measured by a torsion-Langmuir method. The total free-surface vapor pressure was calculated to be
(64) ![]()
or, including the conversion from (atm) to SI units (Pascal):
(65) ![]()
In order to calculate the congruent decomposition rate of GaN the following equation must hold:
(66) ![]()
Both the Ga and the N2 flux can be related to their partial pressures by use of equation (53):
(67) ![]()
(68) 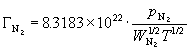
Substituting equations (67) and (68) into (66) results in an expression relating the partial pressures of Ga and N_2:
(69) 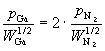
The total pressure is given by Dalton's law of partial pressures as:
(70) ![]()
Rewriting equations (67) and (68) to give the partial pressures in terms of flux, substituting into equation (70), and using equation (66) we obtain the Ga flux as a function of total pressure and temperature:
(71) 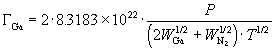
Finally, substitution of equation (65) into (71) gives the Ga flux as a function of substrate temperature alone which is shown in Figure 35, also used in a 1993 paper by N. Newman et. al. (Ref. 44):
(72) 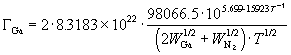
The Ga flux can be converted to the decomposition rate in (ML/s) for 0001 (c-axis) GaN by considering the wurtzite (hexagonal closed-packed) structure using the conversion
(73) ![]()
where a=3.189 Å for GaN. To convert to a decomposition rate in (m/s) we simply use
(74) ![]()
where c=5.185 Å for GaN. The resulting decomposition rate of GaN as a function of temperature is shown in Figure 36.
The free-surface vapor pressure equation (64) was obtained based on non-crystalline wafers manufactures by high pressure pressing of GaN powder. Therefore the decomposition rate should be considered as an upper limit since the surface area was likely to be greater due to its roughness. Also, cracks or pores contribute significantly to the surface area, although according to the authors care was taken to use only crack free wafers. The possibility that the sublimation kinetics were influenced by surface impurities cannot be excluded because neither the original sample purity nor the vacuum provided were good enough to prevent accumulations of impurities at the sample surface if such segregation were chemically favorable, noted by the authors.
On the other hand, as mentioned earlier, Richard C. Schoonmaker et. al. (Ref. 42) found that the rate of vaporization of GaN is markedly enhanced in the presence of Ga. This result suggests that during growth incoming Ga might increase the decomposition rate of GaN. So far no other studies are known giving reliable quantitative data concerning that observation.
In summary the issue of thermal stability of GaN requires much more attention, both in view of growth modeling as well as high temperature GaN devices, especially since material of high crystalline quality is now available. As of now, at substrate temperatures above 800°C the decomposition rate of GaN might become significant during growth, approaching our typical growth rates of about 0.1-0.3um/hour. It is very likely though that significant decomposition does not occur below 900°C due to the arguments given earlier, which seems to be the experience of most groups growing GaN, including ours. More studies are needed to determine the decomposition rate of GaN as a function of temperature, with and without the presence of Ga and NH_3 flux.
An experiment is suggested to investigate the issues addressed above in our MBE system. 4 identical samples of GaN should be prepared on 0001 sapphire substrates using the same sample holder model. A thickness of at least 1µm is being suggested to obtain good crystal quality. Next, the mass of the samples is determined as well as their thickness by Rutherford Backscattering Spectroscopy (RBS). Then all 4 samples are reloaded into the vacuum system using the same sample holder model and subjected to the highest attainable temperature in our system, which we believe is around 880°C (see section on substrate temperature calibration) for the same amount of time. The 1st sample without any flux, the 2nd under Ga flux at the balance point, the 3rd under NH_3 flux, and the last one under both Ga and NH_3 flux at the balance point. The mass of the samples is then again determined, as well as their thickness by RBS. This should give us the decomposition rate at the highest attainable temperature in our system by 2 characterization methods. A more detailed study would repeat the previous steps using different substrate temperatures.
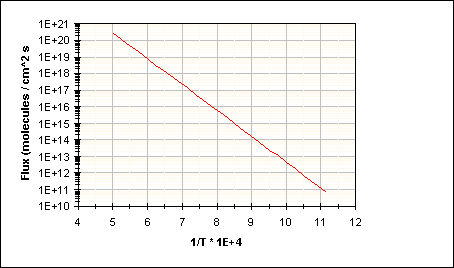
Figure 35: Decomposition Rate of GaN based on Equation (64)
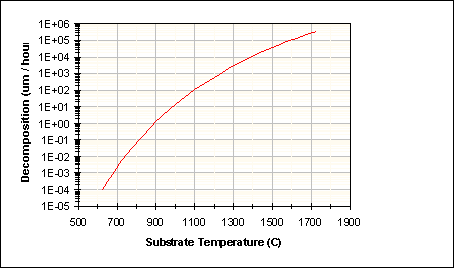
Figure 36: Decomposition Rate of 0001 GaN based on Equation (64)
Table of Contents