Department of Electrical Engineering, University of Minnesota, Minneapolis,
MN 55455
* also, Department of Chemical Engineering and Materials Science, University
of Minnesota, Minneapolis, MN 55455
Journal of Electronic Materials, 26, 3, 1997, p. 272-280
received September 24, 1996; accepted December 2, 1996
+ NOTE ADDED BY AUTHORS AFTER PUBLICATION:
The polarity of the films discussed here was originally believed to
be GaN(0001), also referred
to as GaN(0001)A or Ga-polar. This polarity assignment was based on
references [9, 10, 12].
We later established that these films were of the GaN(0001(bar))
polarity instead. Depending
on nucleation procedure these films might exhibit some inversion domains,
giving rise to a weak (2x2)
reconstruction instead of just the (1x1) pattern observed on unipolar
GaN(0001(bar)).
The surface temperature of a sample in MBE growth is usually determined by using a thermocouple, infra-red (IR) pyrometry, surface reconstruction transitions (Ref. 2), the oxide desorption temperature, or the temperature dependence of the optical band edge (Ref. 3). However, none of these techniques are adept at accurately determining surface temperature in a typical MBE environment suitable for III-N materials, where sample mounting is problematic and temperatures of up to 1000oC are typical. In particular, GaN does not have a congruent sublimation temperature or a well defined oxide desorption temperature. Therefore, simple methods comparable to the GaAs system are not applicable. To address this problem we have developed a novel in situ method to determine GaN surface temperatures using either DMS or RHEED.
To accurately set growth conditions it is important to know the growth rate as a function of surface temperature and fluxes. So far most existing growth rate data on GaN were obtained by ex situ thickness measurements, which even when a superlattice is grown require long growth times for each data point. Some in situ methods were reported, for example, using the period of RHEED intensity oscillations (Ref. 4,5) and IR interference (Ref. 6), but little growth rate data is published. We will present incorporation data obtained using in situ real-time DMS and identify different growth regimes for MBE growth of GaN using NH3.
The next section contains general information about sample preparation and experimental setup. In the Results and Discussion section, DMS and RHEED measurements are presented that describe the main adsorption behavior of Ga on GaN. Also in this section is a discussion combining RHEED and DMS to determine the surface composition of GaN and its control. Based on those observations, techniques are developed in a section entitled Surface Temperature Determination to measure GaN surface temperatures. The measurement of growth rate is then described. In the last subsection, the surface morphology obtained under different growth consitions is discussed. The results are summarized in the last section.
Mounting arrangements involving In bonding were found to be unreliable due to evaporation at the high substrate temperatures used in AlN and GaN growth, resulting in hot spots and eventual loss of the sample. Clips that applied even slight pressure induced unwanted stress onto the sample and caused sapphire to crack during thermal cycling. Instead we used a mount which holds the sample loosely without stress. The coated sapphire substrate was heated directly by radiation from a resistive heater. A thermocouple touched the back of the heater, introducing an offset between the sample surface and thermocouple reading. This offset varied between 50 to 150o, depending upon the growth temperature, as well as from run to run depending on the details of the sample mounting arrangement.
Prior to growth, the substrates were outgassed for several hours at 300oC in the preparation chamber of the MBE system, followed by 1 hour at 500oC in the growth chamber. The NH3 leak valve was set to produce a beam equivalent pressure of 1x10-5 Torr, as determined by a Bayard-Alpert ionization gauge which was positioned close to the sample location during the measurement. Then the substrate temperature was ramped at 100o/min from 500oC to 1000ofor a 15 min surface nitridation. AFM showed that the surface was atomically smooth after this A2O3 nitridation. A 250Å AlN buffer layer was then grown at 1000oC using an Al flux of 1.2x1014 cm-2s-1 (equivalent to 0.10 ML/s AlN) as determined by AlAs RHEED intensity oscillations. RHEED showed a transmission pattern during and after AlN growth. The Ga shutter was then opened to provide a flux of 1.1x1015 cm-2s-1 (or 1.0 ML/s GaN) and the sample temperature was ramped down at 100o/min to 800oC. The Al shutter was closed during the ramp below about 900oC. Finally a 2500Å GaN buffer layer was grown at 800oC. RHEED showed a weakly reconstructed 2x2 pattern after the GaN buffer layer was completed.
A 10 keV electron gun was employed for RHEED measurements of the specular intensity without energy filtering. Measurements were made with the electron beam directed along the GaN <1220> azimuth. The diffracted intensity of the specular beam was measured using a phosphor screen and a photomultiplier tube.
Our DMS apparatus is based on a differentially pumped UTI 100C quadrupole mass-spectrometer mounted on one of the source flanges of the MBE system. A similar apparatus has been used by Tsao et al. (Ref. 7) to study GaAs growth kinetics, and by Jones et al. (Ref. 8) for GaN growth. The solid angle seen by the DMS is limited by the cryoshroud in the growth chamber to subtend only the region around the sample holder. For a few of the measurements to be presented, additional collimation was added in order to limit this region so that only the center of the sample contributed to the desorption signal. This additional collimation reduced the effect of a temperature gradient as well as the background contribution of surrounding cooler surfaces. Since only a small portion of the sample was subtended, the collimation also reduced the signal to noise ratio. Those measurements performed with the additional collimation are indicated.

Fig. 1. A 500 x 500 nm AFM image of an atomically smooth Al2O3 substrates after being cleaned in acetone and methanol, followed by an etch in 1:1 H3PO4:H2SO4. Each step was performed at 65oC for 5 min, followed by a final deionized water rinse and rapid drying with high purity N2. The steps are about 1000 Å in width.
The main point of the measurements to be presented can be understood by considering the adsorption of Ga onto an otherwise inert surface in the absence of NH3. Examine two limits: First, at sufficiently high temperature, an incident Ga flux will produce a steady state Ga coverage that depends on the incident Ga flux and the Ga residence time. In this steady state, the total amount of adsorbed Ga is constant so that the incident flux must equal the desorbing flux. Second, if the substrate temperature is decreased sufficiently, there will be a temperature below which Ga condenses on the surface forming multilayers and/or droplets. Below this temperature the system is not in steady state since the incident flux exceeds the desorbing flux, and Ga is adsorbed continuously. The transition from steady state to condensation is sharp and can be determined by measuring the temperature at which the desorbed Ga flux begins to decrease. For a given incident Ga flux, this transition corresponds to a unique surface temperature well described by equilibrium vapor pressure data.
Employing DMS and RHEED, the adsorption behavior of Ga on Ga terminated GaN will be shown to follow this model over a significant range of temperatures. Over this range the decomposition of GaN can be neglected compared to the incident and desorbing fluxes. Further, above the condensation temperature the difference between the desorbed flux with and without NH3 can be used to measure growth rate. Using RHEED and DMS we will identify the conditions for Ga condensation and present methods to determine surface composition. Employed together these techniques can be used to set growth conditions.
Note that the features illustrated in Fig. 2 are only observed under an excess of Ga. By contrast, under excess NH3 on a sufficiently smooth surface, the diffracted intensity can show damped intensity oscillations (Ref. 5, 4, 13), rather than the behavior of Fig. 2. Continued growth under those conditions, however, eventually leads to a transmission RHEED pattern.
Since similar decreases in the diffracted intensity could result from changes in surface composition, structure, and morphology, it is important to correlate these measurements with other probes. Fig. 3 compares a DMS measurement of the desorbed Ga vs time to the corresponding RHEED measurement made at a temperature below Tc, where Ga condenses on the surface. To improve the signal to noise ratio we did not collimate the mass spectrometer for this set of measurements and therefore an average over the entire surface of the one inch sample is obtained. The RHEED measurement, on the other hand, is more localized, and by moving the beam across the surface we determined that there was an approximately 30o temperature gradient across the sample surface. After opening the Ga shutter at t1, the decrease in the RHEED intensity parallels an increase in the Ga DMS signal to a steady state value at t3. There is a slight decrease in the RHEED intensity after t3 due to increased attenuation of the signal originating from ordered regions. By contrast, the Ga DMS signal is constant after t3. This is consistent if the desorption energies from successive layers are approximately equal. The magnitude of this desorbed Ga is independent of temperature above Tc and then decreases below Tc due to condensation. Below Tc this desorption flux is approximately equal to the flux from liquid Ga following the temperature dependence of the equilibrium vapor. The recovery behaviors of the two signals after the Ga source is shuttered are also correlated to an extent -- the DMS indicates that the increase in the diffracted intensity can be associated with a decrease in the amount of adsorbed Ga and not just to a coalescence of surface adatoms that reduces the step density. The correlation is not exact, however. After the Ga source is shuttered at t4 the RHEED intensity increases slightly until, at t5, there is a rapid increase. By contrast, the DMS signal remains constant during this time and then decreases to a background. This difference in recovery rates would be expected if regions on the sample surface at lower temperature, where there is a larger amount of condensed Ga to desorb, also contribute to the DMS signal. Thus the condensation is seen in both the RHEED and DMS data, though regions sampled can be quite different, as discussed later.

Fig. 2. Intensity variation of the specular RHEED beam along the <1220> azimuth during opening and closing of the Ga shutter in an NH3 flux for (a) above, (b) at, and (c) below the condensation temperature. Curve (a) corresponds to a partial layer of weakly adsorbed Ga and partial nitridation, curve (b) to a complete layer of weakly adsorbed Ga, and curve (c) to Ga condensation.
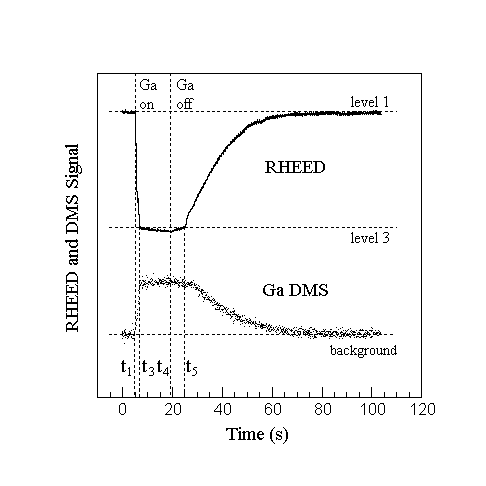
Fig. 3. RHEED and uncollimated Ga DMS signal during Ga accumulation below the condensation temperature. After the Ga shutter is closed at t4 Ga desorption remains constant until part of the GaN surface is exposed at t5. At the same time, the RHEED intensity increases slowly up to t5 where a large change in slope takes place. The slow decrease of the DMS signal after t5 is due to the temperature gradient across the sample, resulting in unsynchronized completion of Ga desorption from the surface.
Figure 4 then compares RHEED and DMS measurements for a surface with no NH3 flux. For these data collimation was not used. A substrate temperature above that for Ga condensation was chosen. The Ga shutter was opened at t1 and the RHEED intensity decreased to a steady state value at t3, after going through a change in slope at t2. The time to reach this steady state depended on the Ga flux.
Since this is a steady state condition, the Ga desorption flux is equal to the incident flux. Further, since there is no NH3 flux, when this steady state is reached, the incident Ga will have had time to react with all of the active nitrogen on the surface. The Ga shutter was then closed at t4, at which time the RHEED intensity increased until it reached at t6 an intensity marked level 2, and the DMS signal simultaneously decreases to a background. Since the final RHEED intensity was independent of the steady state Ga coverage that was achieved during deposition, where the RHEED intensity was below level 2, we interpret this remaining layer to be strongly bound Ga that would make up the bulk termination layer of GaN(0001)A + . It does not leave the surface as easily as the excess Ga, i.e., Ga weakly bound to Ga terminated GaN. At typical growth temperatures, this Ga terminated surface is stable in the absence of either Ga or NH3.
If the surface is subsequently reacted with NH3, the behavior seen in Fig. 2 is obtained and the RHEED intensity rises to the nitrided value of level 1. These observations indicate that there are at least two stable surface terminations possible on GaN(0001)A + -- one is reached after a GaN surface is exposed to an NH3 flux in the absence of Ga (level 1), and the other is obtained after Ga exposure, followed by an anneal in the absence of both NH3 and Ga (level 2).
These two surfaces could subsequently be reacted with NH3 or Ga. Reacting the nitrided surface with Ga gives rise to a hydrogen peak in the DMS, indicating that this surface contains H (Ref. 11, 12). In the case of Ga-termination, the surface can be exposed to a N-supplying flux to examine the reactivity of nitrogen bearing species (Ref. 13).
The picture that emerges is illustrated in Fig. 5 where weakly bound Ga is shown adsorbed on Ga terminated GaN. In this situation, if there were no NH3 or Ga flux present, the excess Ga desorbs, leaving a Ga terminated GaN surface. If now an NH3 flux is provided, the Ga termination layer would react giving a nitrided surface. If then a Ga flux is provided to this nitrided surface, in the absence of NH3, the Ga would react forming GaN and causing hydrogen to desorb. After the nitrogen is consumed, a surface coverage of Ga will built up. If the substrate temperature is above the condensation temperature only a weakly bound Ga layer, likely less than a monolayer, is in steady state, as shown in Fig. 5a. If the substrate temperature is below the condensation temperature for that Ga flux, Ga multilayers and/or droplets will form, as shown in Fig. 5c. The crossover between steady state Ga coverage and condensation is shown in Fig. 5b. Once the Ga flux is stopped, any weakly bound Ga will again desorb to eventually expose the Ga termination layer.

Fig. 4. RHEED and uncollimated Ga DMS signal without NH3 flux above the condensation temperature. Level 1 indicates a nitrided starting surface, while a stable level 2 is reached after Ga exposure, indicating a Ga terminated surface. Note that during Ga exposure the intensity level reaches steady state below level 2 suggesting fractional Ga coverage.

Fig. 5. Behavior of incident Ga on a rigidly attached (strongly bound) Ga termination layer as a function of temperature in the absence of NH3. Above the condensation temperature (a) steady state partial Ga coverage is obtained, and the incident flux equals the desorbing flux. Complete steady state Ga coverage is obtained at a critical temperature (b) below which Ga condensation takes place. Note that the incident flux still equals the desorbing flux. Below this critical temperature (c), Ga multilayer accumulation takes place and the incident flux exceeds the desorbing flux.
(3.1) 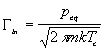
where peq is usually tabulated as (Ref. 14):
(3.2) ![]()
with p given in units of atm., A = 6.754, B = -13984, and C = -0.3413. Here Gammain is the incident Ga flux and T = Tc. Alternatively, both the incident and desorbing fluxes can be expressed more symmetrically by measuring the incident flux in GaN monolayers per second, 1/taui, and by fitting the flux desorbing from the surface in the right side of Eq. (3.1) to an exponential over a range between 500 - 1000oC (also in GaN ML/s). Then Eq. (3.1) becomes:
(3.3) ![]()
where Delta H is the enthalpy of vaporization of Ga and 1/tauo is an attempt frequency. Hence at a given incoming Ga flux, the lowest possible surface temperature to avoid Ga condensation is given by:
(3.4) ![]()
where Delta H is 2.71 eV, tauo = 4.43x10-14 s/ML, and taui is the time it would take to form one ML of GaN on a (0001) plane assuming complete Ga incorporation. Thus once the Ga source is calibrated, the surface temperature can be determined by finding the temperature at which Ga condenses, using either RHEED or DMS.
It should be noted that each technique observes a different region of the surface. Depending on the collimation in our system DMS samples at least a 0.5 cm2 region. The region sampled by RHEED depends on the spot size and incident angle - for a 0.1 mm spot and an incident angle of 2o, RHEED examines a stripe that is 0.1 mm by 6 mm. The latter could be used to map out the temperature gradient over the sample, but drift of the beam could also lead to variations in the measurements. Both methods are compared below.
The RHEED method is illustrated in Fig 2. First one observes the RHEED intensity behavior above the condensation temperature as shown in Fig. 2a. The temperature is then reduced until Ga accumulation occurs as shown in Fig. 2c. The condensation temperature is then found by approaching the condition, from above and below, until a curve similar to Fig. 2b is obtained. The RHEED method was also applied to the case of In on GaN, assuming that the behavior is similar to that of Ga on GaN. The method does not work for Al on GaN, because such high temperatures are needed that GaN decomposes.
Alternatively, the condensation temperature can be found using DMS by monitoring the Ga flux desorbing from the sample. The desorbing Ga flux is monitored without NH3 at an initially high surface temperature above Ga condensation. At such a temperature the incoming flux equals the desorbing flux after steady state is reached. The surface temperature is then reduced until the desorbing flux starts to decrease from its initial constant maximum, indicating that Ga accumulates on the sample surface as shown in Fig. 6. The same procedure can be followed when there is an incident flux of NH3 - the difference is that the Ga signal will be reduced above the condensation temperature due to GaN formation. Nonetheless, the condensation temperature is clearly observed by a discontinuous change in slope as shown in the same figure. No GaN formation was detected below the condensation temperature for high Ga to NH3 flux ratios, and the desorbing Ga flux decreases exponentially according to the Ga vapor pressure equation.
The results for both methods presented here are compiled in Fig. 7, which shows the offset from the thermocouple reading as a function of surface temperature for a typical sample mounting arrangement. Also shown are temperature offsets determined from the melting point of AlSi eutectics mounted on top of a GaN sample, as well as DMS and RHEED measurements using In. The AlSi eutectic temperature was observed by monitoring the temperature dependent emissivity with an IR pyrometer. All data points were taken on the same sample using the same mounting arrangement. Most data points fall within 20oC of a linear fit. Above 750oC the data taken by a collimated DMS using Ga are reproducible to within 1oC. Below this temperature, low Ga fluxes are needed for the measurement and the signal to noise ratio is poor. Note that the data above 750oC are in the range of typical GaN growth temperatures. The larger spread in the RHEED data is due to the combination of a temperature gradient across the sample of about 30o and fluctuations in beam position.
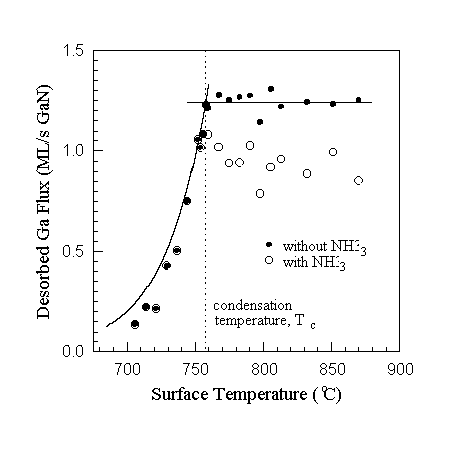
Fig. 6. Condensation temperature determination using collimated Ga DMS with and without NH3 for an incident Ga flux of 1.25 ML/s GaN. Without NH3 the DMS signal remains constant above the condensation temperature, while with NH3 the signal is reduced due to GaN formation. The condensation temperature is found as illustrated by observing the temperature at which the signal drops exponentially without NH3, or by observing the change of slope and subsequent exponential drop with NH3. The solid curve at temperatures below Tc is calculated from Ga vapor pressure data. (The data presented in this figure should not be used for growth rate determination since steady state was not obtained.)

Fig. 7. The offset from the thermocouple reading determined as a function of surface temperature using Ga and In. Employing collimated Ga DMS the data were reproducible to within 1o at typical GaN growth temperatures. The spread in data for In DMS is due to the difficulties in calibrating the source, for Ga and In RHEED due to a temperature gradient across the sample, as well as low fluxes and slow desorption rates at low substrate temperatures. The validity of the method was verified employing the Al-Si eutectic. The large error is due to general problems with IR pyrometry in MBE environments.
We consider deposition onto a surface at a temperature above which Ga condenses. A fraction of the incident Ga incorporates as GaN, and the remainder supplies a steady-state Ga surface concentration which desorbs at the same rate at which it is supplied. Without any NH3 flux all the incident Ga desorbs. We assume that an NH3 flux does not affect the angular distribution of the desorbed Ga flux. In order to obtain the incorporating flux we measure the difference between these two Ga desorption fluxes.
Examples of the Ga desorption signal with and without NH3 are shown in Fig. 8 taken without collimation to improve the signal to noise ratio. Here we measure the change in the desorbed Ga signal when the Ga flux is interrupted since it is somewhat easier to be certain that steady state signals are measured. With \ammonia\ impinging on the surface GaN is grown until steady state conditions are obtained, which in this case was about 15 min. The Ga flux is then interrupted and the DMS signal quickly decreases to a constant background level. We take the drop in the measured mass spectrometer current, Delta Igrowth, to be proportional to the Ga flux leaving the sample surface. Similarly, without NH3, there is a drop in the Ga reference flux, Delta Iref, that is proportional to the incident Ga flux. The growth rate is then computed from
(3.5) 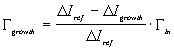
using Gammain as measured by RHEED intensity oscillations and implicitly taking into account the proportionality factor between the measured mass spectrometer current and the desorption flux. Note that at substrate temperatures greater than about 800oC the decomposition of GaN, not included in Eq. (3.5), must also be considered.
Using this method, Figs. 9, 10, and 11 show the GaN growth rate vs Ga flux, NH3 flux, and substrate temperature, respectively. The DMS measurements were taken on the same sample, using a collimator to minimize the effect of the temperature gradient, with 15 minutes of GaN growth between the data points to ensure steady state conditions. Absolute growth rates calculated from the Ga signal were checked independently using a stylus and RBS.
From these three figures a number of important conclusions about the MBE growth of GaN can be made. Fig. 9 shows that the growth is Ga-limited with near unity incorporation up to a certain Ga flux. At higher Ga fluxes the growth rate is NH3-limited and decreases with increased Ga flux, going to zero at the incident Ga flux that determines the condensation condition for this substrate temperature. We have attributed this reduction of growth rate to weakly adsorbed Ga that blocks active Ga terminated sites (Ref. 11, 12). Figure 10 shows that the growth rate in the NH3-limited regime is a linear function of the NH3 flux, up to the flux where near unity Ga incorporation is obtained, beyond which the growth becomes Ga-limited. Growth rates as a function of substrate temperature are shown in Fig. 11. The growth rate decreases with decreasing substrate temperature going to zero at the condensation condition where multilayers of Ga cover the surface.
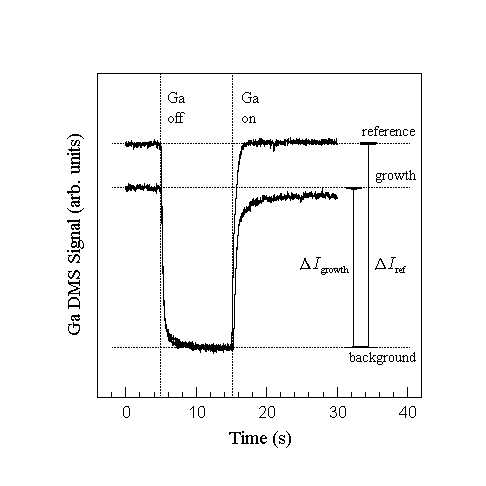
Fig. 8. Typical uncollimated Ga DMS signal during closing and opening of the Ga shutter. The growth and reference signal is obtained by measuring the rapid change in signal after closing the Ga shutter to avoid contributions of the slowly varying background signal from other cooler parts of the system. Growth rates are obtained by calculating the incorporation fraction from the signals and multiplying by the known incident flux. Note that the growth rate is obtained by closing the Ga shutter instead of opening to ensure steady state after 15 min of growth.

Fig. 9. GaN growth rate as a function of Ga flux at constant NH3 flux of 1x10-5 Torr and surface temperature of 785oC. Two growth regimes can be identified, Ga-limited and NH3-limited. Near unity incorporation of Ga is obtained during Ga-limited growth. During NH3-limited growth the growth rate reduces as a function of Ga flux, approaching zero at the flux required for condensation at this temperature. This reduction is attributed to weakly adsorbed Ga blocking active Ga terminated GaN sites.
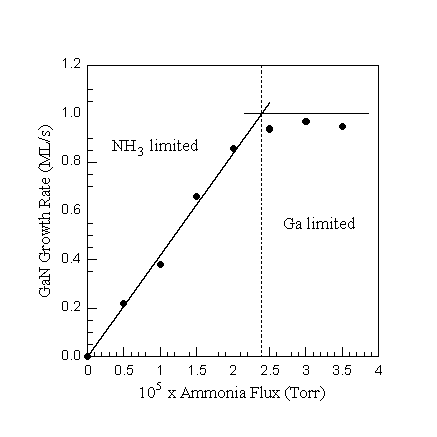
Fig. 10. GaN growth rate as a function of NH3 flux at constant Ga flux of 1.0 ML/s (GaN) and surface temperature of 785oC. Both growth regimes can be identified, NH3-limited and Ga-limited. In the NH3-limited regime the growth rate is a linear function of the NH3 flux, while in the Ga-limited regime near unity Ga incorporation is obtained.

Fig. 11. GaN growth rate as a function of surface temperature at constant Ga flux of 1.0 ML/s (GaN) and NH3 flux of 1x10-5 Torr. The growth rate reduces towards the condensation temperature, attributed to weakly adsorbed Ga blocking active Ga terminated GaN sites.
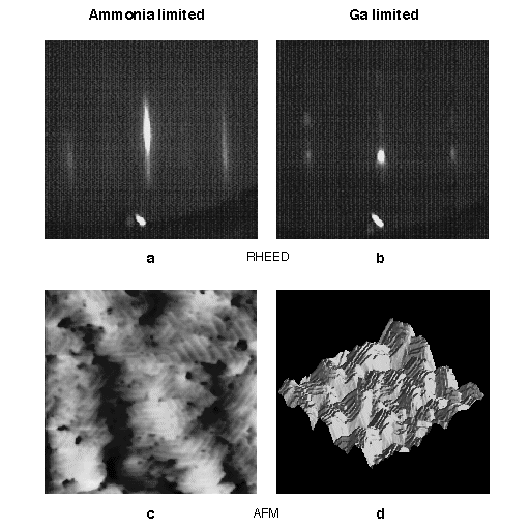
Fig. 12. RHEED patterns with the incident beam along the <1220> azimuth and AFM images for both NH3 and Ga-limited growth. The AFM scans cover an area of 2 x 2 microns with a z-range of 50Å and 1000Å, respectively. NH3-limited growth gives rise to a weakly (2 x 2) + reconstructed RHEED pattern (a) and AFM shows atomic steps on islands (c). Ga-limited growth results in a RHEED pattern with a strong transmission component (b) and AFM indicates that this is due to facets (d).
2 A.M. Dabiran and P.I. Cohen, J. Cryst. Growth 150, 23 (1995).
3 W.S. Lee, G.W. Yoffe, D.G. Schlom, J.S. Harris, J. Cryst. Growth, 111, 131 (1991)
4 Y. Moriyasu, H. Goto, N. Kuze, M. Matsui, J. Cryst. Growth 150, 916 (1995)
5 L.K. Li, Z. Yang, W.I. Wang, Electron. Lett. 31, 2127 (1995)
6 S. Nakamura, Jpn. J. Appl. Phys. Part 1, 30, 1620 (1991)
7 J.Y. Tsao, T.M. Brennan, and B.E. Hammons, Appl. Phys. Lett. 53, 288 (1988)
8 C.R. Jones, T. Lei, R. Kaspi, and K.R. Evans, Proc. 1995 Fall MRS Mtg. (Pittsburgh, PA: Mater. Res. Soc.).
9 F.A. Ponce, B.S. Krusor, J.S. Major, Jr., W.E. Plano, and D.F. Welch, Appl. Phys. Lett. 67, 410 (1995).
10 B. Daudin, J.L. Rouviere, and M. Arlery, Appl. Phys. Lett. 69, 2480 (1996).
12 D.E. Crawford, Ph.D. Thesis, University of Minnesota, Minneapolis, MN 55455.
13 A.M. Johnston, Ph.D. Thesis, University of Minnesota, Minneapolis, MN 55455
14 CRC Handbook of Chemistry and Physics, 71st Edition, Editor-in-Chief David R. Lide, (Boca Raton:CRC Press, 1990), p. 5-70
Back to my home page